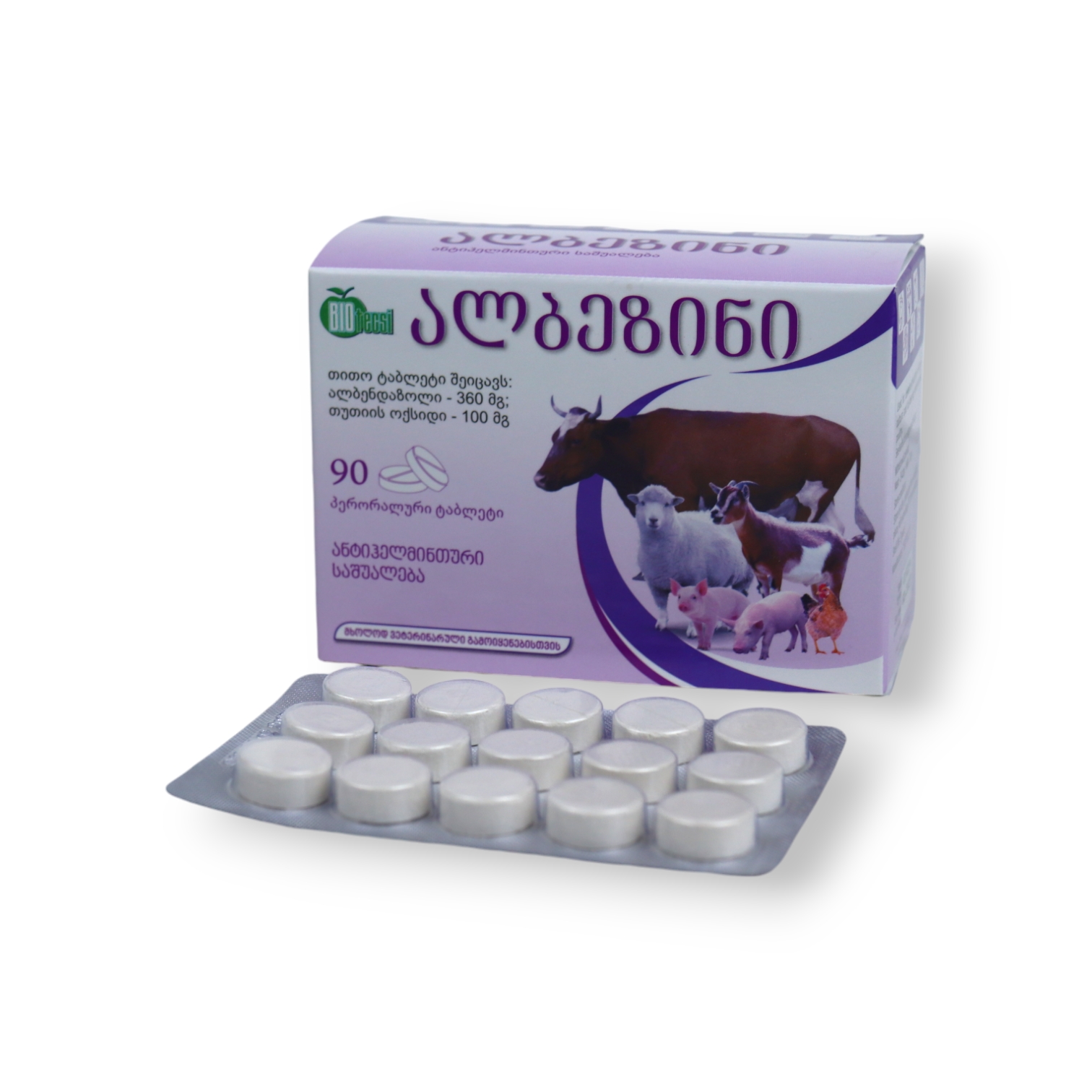
Albezin
Albezin
Tablet for oral administration – Veterinary use
Pharmacotherapeutic group: Anthelmintic
Target species:
Cattle, sheep, goats, pigs, horses, donkeys, mules, rabbits, fur-bearing animals, and poultry
Composition (per tablet):
-
Albendazole 360 mg
-
Zinc oxide 100 mg
Description:
White or nearly white tablet, one side scored, the other marked “BIO”; white specks may be visible.
Form of Release:
-
90 tablets in blister packs
Pharmacological Properties:
Albendazole, a benzimidazole carbamate derivative, has a broad-spectrum anthelmintic effect. It is effective against adult and larval forms of nematodes, cestodes, and trematodes and possesses ovicidal activity that prevents contamination of grazing areas and barns.
Albendazole inhibits microtubule polymerization, disrupting glucose transport and causing functional failure of the parasite’s microtubular system, leading to death and expulsion.
Zinc has antiseptic, adsorptive, anti-inflammatory, astringent, and disinfectant properties. It participates in various metabolic processes including protein and collagen synthesis. It promotes healing and epithelial regeneration in gastrointestinal lesions caused by parasites.
The combination of zinc and albendazole reduces albendazole side effects (e.g., liver dysfunction, leukopenia, hypersensitivity).
Toxicity:
Albezin tablets are classified as low-risk (hazard class IV) and are well tolerated at recommended doses.
Pharmacokinetics:
Albendazole is better absorbed than other benzimidazoles. About 47% of the dose is excreted in urine as metabolites within 9 days. Maximum plasma concentration of active metabolites occurs within 20 hours after oral administration.
Zinc is absorbed from the small intestine and excreted mainly via feces and urine.
Indications for Use:
Used for deworming in farm animals, fur-bearing animals, and poultry. Effective against:
-
Nematodoses (gastric-intestinal and lung parasites): Haemonchosis, Bunostomosis, Esophagostomosis, Nematodiriasis, Ostertagiasis, Cooperiasis, Strongylatosis, Trichostrongyliasis, Hyostrongylosis, Parascaridiasis, Trichocephalosis, Toxocariasis, Uncinariosis, Ascaridiasis, Heterakidosis
-
Cestodoses: Monieziasis, Avitellinosis, Thysanieziasis
-
Trematodoses: Fascioliasis, Dicrocoeliasis, Paramphistomiasis
Dosage and Administration:
Given orally, with or without prior fasting, individually or in groups (poultry), with feed as a single dose. For fur animals and poultry, administered twice.
-
Cattle:
-
Nematodes (GIT/lung): 1 tab/50 kg
-
Cestodes: 1 tab/35 kg
-
Trematodes: 1 tab/25–35 kg
-
-
Sheep, goats:
-
Nematodes & cestodes: 1 tab/35–70 kg
-
Trematodes: 1 tab/45 kg
-
-
Pigs: 1 tab/20–35 kg
-
Horses, donkeys, mules: 1 tab/50 kg
-
Fur-bearing animals, rabbits: 1 tab/7–10 kg
-
Poultry:
-
GI nematodes: 1 tab/25–30 kg
-
Lung nematodes: 1 tab/30–35 kg
-
Once daily for 2 days
-
Side Effects:
None observed even at triple therapeutic dose.
Contraindications:
Hypersensitivity to components.
Do not use in debilitated animals, acute fascioliasis, or during the first third of pregnancy.
Withdrawal Period:
-
Meat: 14 days after treatment
-
Milk: 3 days after treatment
Storage Conditions:
Store in a dry, dark place at temperatures not exceeding 25°C.
Shelf Life: 3 years
Manufacturer: LLC “Biotecsi,” Tbilisi, Georgia, 8 Yumashvili St.