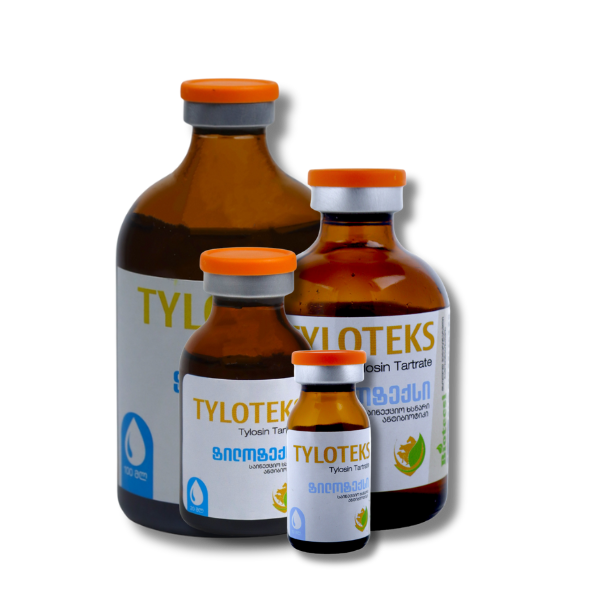
Tyloteks
Tyloteks
Form of medicine: Injectable solution
Pharmacological group: Systemic antibacterial agent, antibiotic;
Target species: Cattle, sheep, goats, pigs, dogs, cats, poultry.
Composition: Each 1 ml of injectable solution contains the active substance: tylosin tartrate - 100 mg.
Description: Transparent yellow liquid with a characteristic odor.
Packaging: Available in 10 ml, 20 ml, 50 ml, and 100 ml in dark-colored glass vials, hermetically sealed with a rubber stopper, aluminum cap, and a plastic cover for first-time opening control.
Pharmacological properties: Tylosin tartrate is an antibiotic from the macrolide group with broad-spectrum bacteriostatic activity, effective against gram-positive bacteria and some gram-negative microorganisms. Its mechanism of action involves inhibition of protein synthesis in bacterial cells through the formation of a complex with the 50S ribosomal subunit. After intramuscular injection, the antibiotic is rapidly absorbed, reaching maximum concentration in tissues about 1 hour after administration. The therapeutic level of the antibiotic remains in the body for 20-24 hours. It is excreted mainly in urine and bile, and in lactating animals, it is excreted in milk. It is classified as a low-risk substance based on its effect on the organism (risk class 4, according to state standard 12.1.007-76).
Indications:
- Cattle: Respiratory tract diseases, diphtheria, mastitis, metritis, secondary complications of viral diseases;
- Sheep, goats: Infectious agalactia, goat pneumonia;
- Pigs: Endemic pneumonia, mycoplasma arthritis, swine erysipelas, dysentery, gastroenteritis, bacterial etiology diseases;
- Dogs, cats: Gastrointestinal tract, respiratory, and urinary tract bacterial diseases;
- Poultry: Respiratory mycoplasmosis, infectious sinusitis in turkeys, chronic respiratory infections in chickens and turkeys, infectious rhinitis in chickens (contagious cold), spirochetosis in chickens and turkeys, necrotizing enteritis.
Side Effects:
No side effects or complications were observed when used at the recommended doses. Very rarely, allergic reactions may occur in pigs, such as erythema, itching, respiratory symptoms, mild swelling, and minor prolapse of the rectum, which disappear quickly after discontinuing the drug.
Contraindications:
Increased individual sensitivity to tylosin. Not for use in horses. Avoid simultaneous use with tiamulin, clindamycin, levomycetin, penicillin, cephalosporins, lincomycin due to reduced antibacterial effects.
Dosage and administration:
The drug is administered intramuscularly once a day for a course of 3-5 days:
- Cattle, goats, sheep: 1 ml per 10-20 kg of body weight;
- Pigs: 1 ml per 10-50 kg of body weight;
- Dogs, cats: 1 ml per 10-20 kg of body weight;
- Poultry: 0.1 ml per 1 kg of body weight.
Attention: The maximum volume for one injection site is 5 ml for pigs and 10 ml for cattle.
Special precautions:
The drug is compatible with sulfonamides, nitrofurans, erythromycin, and spectinomycin. When handling the drug, general hygiene rules and safety measures for working with medicines should be followed. Do not drink, smoke, or eat while working with the drug. After use, wash hands with warm water and soap. Those with hypersensitivity to tylosin should avoid direct contact. If the drug comes in contact with skin or mucous membranes, rinse immediately with plenty of running water. In case of allergic reactions or accidental self-injection, seek immediate medical attention.
Use during pregnancy and lactation:
Use with caution during pregnancy and lactation under veterinary supervision.
Withdrawal periods:
- Cattle, sheep, goats, pigs: Slaughter is allowed 8 days after the last dose;
- Poultry: 3 days after the last dose;
- Eggs: Safe for human consumption if used according to the manufacturer's instructions; egg waiting period is 0 days;
- Milk: Safe for human consumption 4 days after the last dose.
Storage conditions:
Store in a dry, cool place, protected from light, at a temperature not exceeding 20°C. Keep out of reach of children. For veterinary use only.
Expiration date:
2 years from the production date. Do not use after the expiration date printed on the vial.
Manufacturer:
LLC "Bioteksi," Georgia, Tbilisi, Yumashvili St. #8